advanced technology eliminates need for invasive biopsies
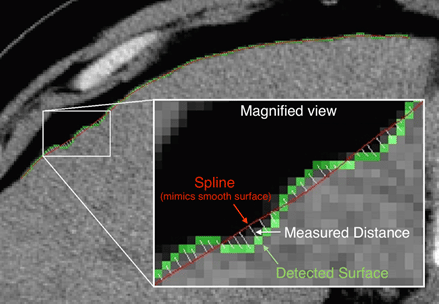
With higher stages of chronic liver disease, the liver surface becomes more nodular due to increasing amounts of liver fibrosis. Liver surface nodularity (LSN) is visible on routine abdominal CT images. Using the advanced LSN software, radiologists can now non-invasively and rapidly measure LSN from routine abdominal CT images and generate a Liver Score, which can be used to evaluate the severity of chronic liver disease, eliminating the need for a biopsy.
aycan workstation is an FDA 510(k) cleared, vendor-neutral, highly functional advanced image-processing tool and DICOM PACS workstation for conventional, multi-slice, and other image reading. aycan continually develops/adds clinical and workflow plug-ins to further enhance the capabilities of aycan workstation, which, in addition to the new LSN software, includes hanging protocols, and FusionSync (automatic image fusion plug-in).
ABOUT aycan
With a focus on vendor-neutral integration and service, aycan is a leading global provider of solutions for viewing, printing, sharing, and storing medical images and information that improve workflow efficiency and drive down costs. aycan works with other industry innovators such as Apple and Xerox, whose quality products are part of their solutions. aycan supports worldwide customers with U.S. headquarters in Rochester, New York, and European headquarters in Wuerzburg, Germany. The company is privately held. www.aycan.com